Dental Enamel
The crown of the tooth is covered with a very hard layer of enamel. The enamel is thinnest at the neck of the tooth and increases in thickness up to the incisal or occlusal surface of the tooth. On the occlusal surface, the enamel layer is up to 2 mm thick.
In healthy teeth, the enamel is virtually colorless; it has a slight bluish tinge, making it appear translucent. Therefore, the particular coloring of a tooth is not determined solely by the inherent color of the enamel but by the shade of the underlying dentin. The yellowish dentin is more visible through the thin layer of enamel at the neck of the tooth. This is why the neck looks darker, while at the incisal edge the tooth appears to have a lighter, almost transparent consistency.
Enamel is the hardest substance in the human body and extremely resistant to mechanical stresses and strains. Being composed of inorganic substances gives enamel very high resistance to chemical influences. However, this hardest dental substance is also the most brittle in the human body and, in line with its mineral density, is harder and more brittle at the surface than in the deeper layers.
Mature enamel differs in its composition and characteristics from the other hard substances of the teeth and bones. It contains 98% inorganic and only 2% organic materials. The crystalline part of enamel is made up of hydroxyapatites of calcium phosphate. It may also contain carbonates such as manganese carbonate, sodium carbonate, calcium carbonate, and fluoride carbonate.
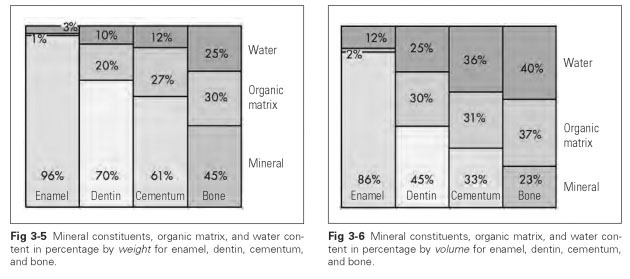
Hydroxyapatite has the chemical formula Ca10(PO4)6(OH)2, although the hydroxyl groups can often be substituted by fluoride and chlorine. The fluoride content hardens the dental enamel and varies widely. The fluoride concentration is far greater (20 times) at the surface than in the lower layers and fluctuates further depending on the amount of fluoride consumed in the diet, drinking water, and toothpaste. In its immature state, dental enamel has 50% water content, but this rapidly decreases as the enamel matures, leaving a residue of only 4% by weight, which accounts for 14% of its volume (Figs 3-5 and 3-6). A quarter of this residual water is found in the organic substance of enamel and makes up about 1% of its weight (2% of its volume). The other residual water is bound to the apatite crystals in the form of hydroxyl groups.
The organic substance of enamel comprises proteins, carbohydrates, and lipid compounds. The enamel layer determines the outer shape of the crown of the tooth. However, these shapes are not random but determined by the natural laws of form and function. Every enamel crest, cusp, or fissure therefore has a very specific task. When tooth substance is formed during growth, the ameloblasts (enamel-forming cells; adaman-toblasts) are the building blocks for enamel, and these give the specific shape to the teeth during development. Shortly after eruption, there is a very thin membrane (1 mm) on the enamel surface. This is known as the enamel cuticle (cutic-ula dentis). This membrane is very resistant but is soon worn away; the enamel cuticle remains longer at the neck of the tooth. No new enamel is formed once the tooth has erupted. The enamel layer, once developed, has to last for the lifetime of the tooth (ie, the durability of the enamel largely determines the durability of the tooth).
The enamel layer has several functions. It protects the dentin core of the tooth against thermal and chemical influences. The purpose of its specific hardness is to resist mechanical stresses during chewing, although older teeth will show a certain amount of wear at the incisal and occlusal surfaces (abrasion). The protective effect of enamel also extends to bacterial influences.
Despite its density, enamel is permeable to liquids. This means that pigments, water, and alcohol can flow relatively freely through enamel (water penetrates enamel to a depth of about 4 mm within 24 hours). This is why chemical changes that help to maintain or alter the composition of the enamel can be initiated in enamel.